Essential Question: How can we determine if water is too contaminated to drink?
Lesson Summary:
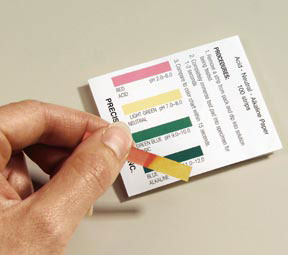
In part 1 of the laboratory procedure we tested the pH( a measure of acidity) for eight different beverages. The lower the pH the more acidic the substance. The higher the pH, the more basic a substance is. The scale ranges from 1 to 14, with 7 being neutral. Students found neutral pHs for water fountain water, milk, fiji, and poland spring. Coke and vitamin water were highly acidic and possibly dangerous and could possibly lead to health problems by disrupting pH homeostasis in the body.
For part 2 each lab group used a water testing kit to record levels of copper, iron, chlorine, nitrogen, and hardness in the water. The class concluded that the low level of contaminants in the water fountain water was safe enough to drink. Alternatively, the high levels of nitrogen in the compost water was dangerous to consume.
Lesson Summary:
In part 1 of the laboratory procedure we tested the pH( a measure of acidity) for eight different beverages. The lower the pH the more acidic the substance. The higher the pH, the more basic a substance is. The scale ranges from 1 to 14, with 7 being neutral. Students found neutral pHs for water fountain water, milk, fiji, and poland spring. Coke and vitamin water were highly acidic and possibly dangerous and could possibly lead to health problems by disrupting pH homeostasis in the body.
For part 2 each lab group used a water testing kit to record levels of copper, iron, chlorine, nitrogen, and hardness in the water. The class concluded that the low level of contaminants in the water fountain water was safe enough to drink. Alternatively, the high levels of nitrogen in the compost water was dangerous to consume.
 RSS Feed
RSS Feed